
I've been asked before what's the difference between soap and detergent. After giving my proof-readers a little taste of chemistry, I noticed their eyes began to look like doughnuts... glazed. To hopefully make things more understandable, I have included pictures that my kids I drew. OK... yes, I drew them.
Surfactants
Both soaps and detergents are known as surfactants (short for surface-active agents). Surfactant molecules contain a lipophilic (fat-loving) end that attaches grease dirt and a hydrophilic (water-loving) end which makes the molecule dissolve in water. The electrical charge on the water-loving end of the molecule distinguishes between the different types of surfactants. Surfactants come in four different types: anionic, nonionic, cationic, and amphoteric.
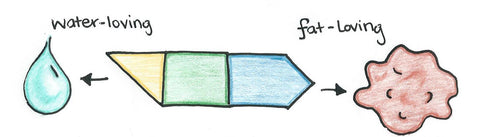
Soap
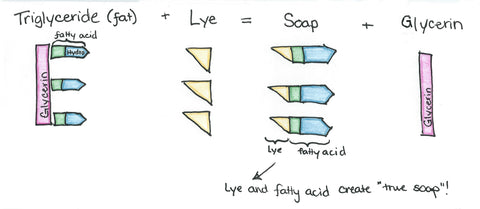
Soap, by definition, is the product of a fat and an alkali -- usually sodium or potassium hydroxide. Fat, made of triglycerides, is composed of three fatty acids (hence the prefix "tri-") connected to one glycerin (hence "glyceride"). There are many types of triglycerides; each type consists of its own particular combination of fatty acids.

It is the fatty acid part that reacts with sodium or potassium hydroxide that creates a soap with the glycerin left behind.
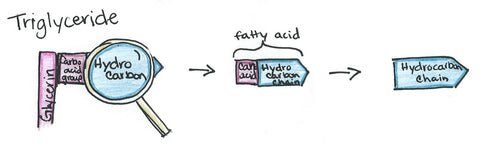
Detergent
Detergents are created in a multi-step process -- making a completely synthetic substance. It's literally called a "slurry mixture" when being developed.
It contains a hydrocarbon (which is a fraction of a fatty acid, which is a part of a triglyceride/whole fat) from either a petrochemical, derived from petroleum, or an oleochemical, derived from fat and oils. The hydrocarbon will become fat-loving end of the surfactant.
Other chemicals, such as sulfur trioxide, sulfuric acid, and ethylene oxide, are used to produce the water-loving end of the surfactant molecule. The hydrocarbon plus the other chemicals creates the new, synthetic acid which is ready to be mixed with another agent.
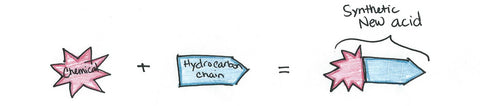
Sometimes it is mixed with ethylene oxide to create a nonionic surfactant. Other times it is mixed with an alkali - usually sodium or potassium hydroxide - to make an anionic surfactant. However, the differences in detergent ions is going beyond the scope of this post.
I'd like to point out that the creation of detergents does not include glycerin which is why we use conditioner after shampoo and lotion after body wash. They not only remove oils which are our protective barrier, but also fail to moisturize (via glycerin) nor leave a moisturizing barrier behind. Additionally, the Journal of the American College of Toxicology (1983, Vol. 2, No. 7), has noted that detergents are skin irritants as well.
In labeling, detergents may not be called soap. It does not contain a full fatty acid (only a hydrocarbon) and it may or may not even use an alkali. It's completely synthetic. It does not fit the definition of a soap; it's a detergent. However, this doesn't stop clever marketers from calling their product "beauty bar," "anti-aging moisturizing bar," or "deodorant bar." This also doesn't stop marketers for wanna-be-natural products to say their sodium lauryl sulfate is all-natural because the hydrocarbon came "from coconut oil."
Often times people use the word "soap" when referring to anything that cleans -- for example, "dish soap," "hand soap," "anti-bacterial soap," etc. However, this can be detrimental for one's understanding because, unlike real soap which mechanically removes dirt AND kills bacteria, detergents can only physically remove dirt. You can learn more about detergents in my next post!
Thanks for reading!




ARE YOU A VICTIM OF INVESTMENT OR NFT SCAM? DO YOU DESIRE CREDIT REPAIR (ALL BUREAUS)? SCHEDULE A MEETING WITH AN ETHICAL HACKER ASAP TO GET STARTED.
Workmanship fee is to be settled AFTER recovery completion.
Let us show you the art of Ethical Hacking….!
Asore Hack Corp. is a financial regulator, PRIVATE investigation and funds recovery body. We specialize in cases as regards ETHICAL HACKING, CRYPTOCURRENCY, FAKE INVESTMENT SCHEMES and RECOVERY SCAM. We are also experts in CREDIT REPAIR, we analyze what’s impacting your score.
All software tools needed to execute RECOVERIES from start to finish are available in stock.
Kindly NOTE that the available tools does NOT apply to CREDIT FIX.
Be ALERT to FALSE reviews and testimonies on the internet, the authors and perpetrators unite to form a syndicate.
Contact our team as soon as you can via the email address below to book a mail meeting with an ethical hacker.
asorehackcorp (@) gmail (.) com
Transparency is our watchword !
Stay Safe out there !