
I've been asked before what's the difference between soap and detergent. After giving my proof-readers a little taste of chemistry, I noticed their eyes began to look like doughnuts... glazed. To hopefully make things more understandable, I have included pictures that my kids I drew. OK... yes, I drew them.
Surfactants
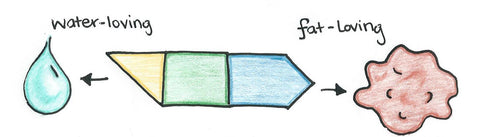
Both soaps and detergents are known as surfactants (short for surface-active agents). Surfactant molecules contain a lipophilic (fat-loving) end that attaches grease dirt and a hydrophilic (water-loving) end which makes the molecule dissolve in water. The electrical charge on the water-loving end of the molecule distinguishes between the different types of surfactants. Surfactants come in four different types: anionic, nonionic, cationic, and amphoteric.
Soap
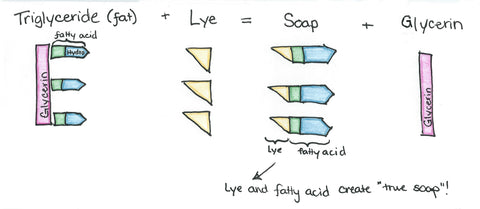
Soap, by definition, is the product of a fat and an alkali -- usually sodium or potassium hydroxide. Fat, made of triglycerides, is composed of three fatty acids (hence the prefix "tri-") connected to one glycerin (hence "glyceride"). There are many types of triglycerides; each type consists of its own particular combination of fatty acids.

It is the fatty acid part that reacts with sodium or potassium hydroxide that creates a soap with the glycerin left behind.
Detergent
Detergents are created in a multi-step process -- making a completely synthetic substance. It's literally called a "slurry mixture" when being developed.
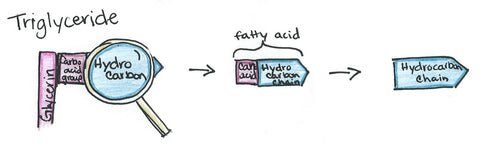
It contains a hydrocarbon (which is a fraction of a fatty acid, which is a part of a triglyceride/whole fat) from either a petrochemical, derived from petroleum, or an oleochemical, derived from fat and oils. The hydrocarbon will become fat-loving end of the surfactant.
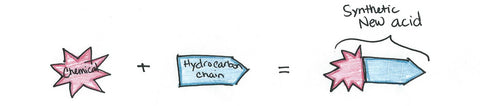
Other chemicals, such as sulfur trioxide, sulfuric acid, and ethylene oxide, are used to produce the water-loving end of the surfactant molecule. The hydrocarbon plus the other chemicals creates the new, synthetic acid which is ready to be mixed with another agent.
Sometimes it is mixed with ethylene oxide to create a nonionic surfactant. Other times it is mixed with an alkali - usually sodium or potassium hydroxide - to make an anionic surfactant. However, the differences in detergent ions is going beyond the scope of this post.
I'd like to point out that the creation of detergents does not include glycerin which is why we use conditioner after shampoo and lotion after body wash. They not only remove oils which are our protective barrier, but also fail to moisturize (via glycerin) nor leave a moisturizing barrier behind. Additionally, the Journal of the American College of Toxicology (1983, Vol. 2, No. 7), has noted that detergents are skin irritants as well.
In labeling, detergents may not be called soap. It does not contain a full fatty acid (only a hydrocarbon) and it may or may not even use an alkali. It's completely synthetic. It does not fit the definition of a soap; it's a detergent. However, this doesn't stop clever marketers from calling their product "beauty bar," "anti-aging moisturizing bar," or "deodorant bar." This also doesn't stop marketers for wanna-be-natural products to say their sodium lauryl sulfate is all-natural because the hydrocarbon came "from coconut oil."
Often times people use the word "soap" when referring to anything that cleans -- for example, "dish soap," "hand soap," "anti-bacterial soap," etc. However, this can be detrimental for one's understanding because, unlike real soap which mechanically removes dirt AND kills bacteria, detergents can only physically remove dirt. You can learn more about detergents in my next post!
Thanks for reading!




VERIFIED CRYPTOCURRENCY RESTORATION/FOLKWIN EXPERT RECOVERY.
As a designer, I’m always on the lookout for new tools to improve my workflow. So, when I saw an ad on LinkedIn for a “revolutionary” design software, it seemed like the perfect opportunity. The ad promised to streamline my design process with amazing features at an unbeatable price. Without a second thought, I clicked the link and made the purchase. But soon after buying the software, I realized it was a scam. The software never worked as promised, and when I tried to reach out for support, the company vanished without a trace. I was left feeling frustrated and powerless. 5,000 NZD, a significant amount for anyone, especially a freelancer, was gone, and there was no way to get it back… or so I thought. I was at a loss for what to do next. That’s when I discovered FOLKWIN EXPERT RECOVERY. At first, I wasn’t sure if they could help. Could anyone actually recover money lost to online scams? But, desperate and with no other options, I decided to reach out. I contacted FOLKWIN EXPERT RECOVERY, they explained the process in detail, reassured me that they would do everything in their power to recover my funds, and kept me updated every step of the way. To my amazement, just two days after I reached out, they successfully recovered 4,800 NZD of the 5,000 NZD I had lost. The relief I felt when I saw that money back in my account was indescribable. It was like a huge weight had been lifted off my shoulders. Since then, I’ve made it a point to share my experience with other designers in the community. I want to raise awareness about the scams that are out there and remind others to be cautious when browsing for new software. Scammers can target anyone, even professionals like us. But I also want to let fellow designers know that if they do fall victim to fraud, they don’t have to give up. FOLKWIN EXPERT RECOVERY is there to help. Now, I always tell others: Be skeptical of ads that seem too good to be true, especially on platforms like LinkedIn. But if you do get scammed, there’s hope. Don’t hesitate to contact FOLKWINEXPERTRECOVERYTECH-CENTER. CO M, (WhatsApp): +1 (740)705-0711. They helped me get my money back in just two days, and they can help you too.
Warm greetings,
Mr. Gilbert Jonathan